▶ Plasmapp is the world’s first clinical validation of medical implant surface treatment technology using vacuum plasma technology.
▶ Harvard faculty publishes a paper in the journal Bioengineering on preclinical research on dental implants. ▶ A paper was published in the journal Bioengineering on preclinical research on plastic surgery implants at a domestic university hospital. ▶ Expanded commercialization by launching a new implant surface treatment machine model at a recent plastic surgery conference held in Seoul.
Plasmapp (405000) is a medical implant surface treatment technology that published preclinical research results on human acellular dermal matrices (hADM) used in plastic surgery and orthopedics in the SCI-level international professional journal 'Bioengineering (IF: 5.046)' on April 8.
Plasmapp developed plasma optimization conditions through joint research with KAIST, and the in vitro research results conducted at the Plasmapp Research Institute have already been published in SCI-level international professional journals in 2022. Harvard University professors published the results of preclinical research on dental implant fixtures in the international journal 'Bioengineering' on October 11 last year, and this time, the same research results showing the clinical effectiveness of plasma technology for plastic surgery implants were published. It was published in an international academic journal and was evaluated as a case that technically proved that Plasmapp's technology can be commercialized in various medical implant markets.
Plasmapp developed a commercial product to prove marketability as well as technical verification of the implant surface treatment machine, and presented the product at APS 2024, a plastic surgery conference held for two days from April 6th to 7th at the Grand Intercontinental Seoul Parnas. The new product (model name: ACTILINK reborn S) was unveiled for the first time, drawing attention from academia and industry. This is because research results show that the results of reconstructive surgery using implants used in plastic surgery can be improved through plasma surface treatment technology, and Plasmapp's surface treatment products are already actively used in the global dental market, showing their reliability.
Youbong Lim, CEO of Plasmapp, said, “Plasmapp has developed innovative plasma technology and applied for about 200 patents, and is pursuing the commercialization of plasma products that enable safer and more effective treatment in the medical industry in the global medical device market.”, “We have invested intensively in technology and product development in 2023, and will reap the benefits of sales and profitability this year,” he said, expressing confidence in this year’s business performance.
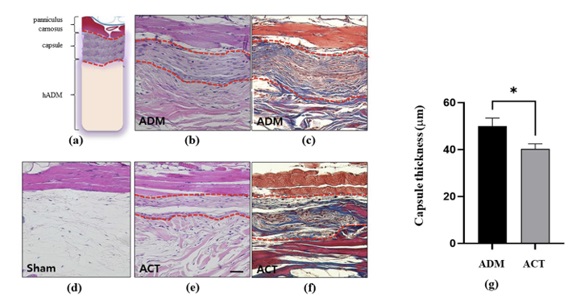
<Some excerpts from papers published in the Bioengineering journal. Photo provided by Plasmapp>

<Photos of Plasmapp’s new surface treatment machine and participation in the APS exhibition. Photo provided by Plasmapp>
| 
