▶ Preclinical research with professors from the University of Rome, Italy, and others led to the publication of a paper in March of this year and approval of a clinical study on May 8th.
▶ First in the world to proceed with medical device certification in the US and Europe, with the first clinical trial starting in Europe.
Plasmapp (405000) announced on May 8th that it received clinical study approval to move forward with a clinical study following the publication of a preclinical study using Plasmapp's surface treatment machine (brand: ACTILINK) with faculty from the Universities of Roma, Genoa and Torino in Italy.
Plasmapp has developed a medical implant surface treatment device using vacuum plasma technology and is rapidly moving toward commercialization, having received all major global electronics certifications. Plasmapp is the first company in the world to demonstrate the clinical safety and efficacy of its plasma surface treatment technology and is pursuing global medical device certifications. Starting with the Japanese medical device certification in April last year, the company signed a supply contract with YOSHIDA, one of the largest distributors in Japan, and is rapidly increasing sales in the Japanese market. With the approval of the clinical study in Europe, the safety has been verified first and foremost, and the clinical efficacy has been demonstrated through recent multiple papers, increasing the visibility of the European medical device certification.
"We are developing the world's first innovative technologies and promoting their commercialization in the global medical device market, and we are already promoting cooperation with global implant manufacturers in Korea," said Youbong Lim, CEO of Plasmapp. "If we obtain the world's first European medical device certification through this clinical trial approval, we are already planning to cooperate with many European manufacturers, which will allow us to increase our sales growth faster than planned."
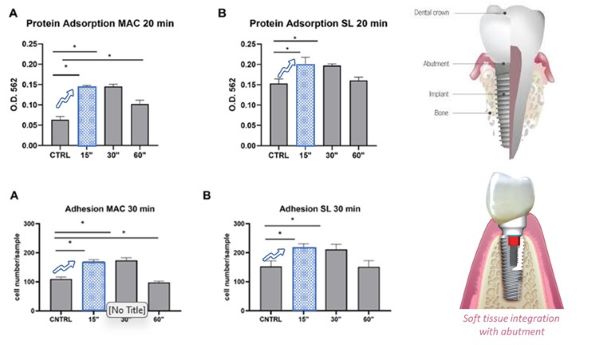
<Image of the paper on clinical efficacy published by the Italian faculty in March and related photos>
| 